Atoms are the fundamental building blocks of matter, the smallest units that retain the properties of an element. Though they are incredibly tiny, their structure is intricate and fascinating. To truly appreciate the complexity of the universe, one must delve into the world of atoms and their subatomic components: protons, neutrons, and electrons.
What is an Atom?
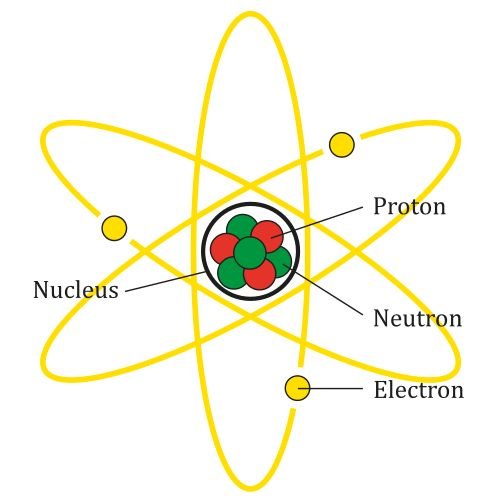
An atom consists of a central nucleus surrounded by a cloud of electrons. The nucleus itself is made up of protons and neutrons. While the concept of atoms has been around since ancient Greek philosophers like Democritus, it wasn’t until the early 20th century that scientists like Ernest Rutherford and Niels Bohr began to unravel the atom’s structure.

Protons: The Positive Charge
Protons are positively charged particles found within the atomic nucleus. Each proton carries a charge of +1. The number of protons in an atom’s nucleus determines the element to which the atom belongs. For example, a hydrogen atom has one proton, while a carbon atom has six. This number of protons is referred to as the atomic number.
Neutrons: The Neutral Force
Neutrons are neutral particles, meaning they carry no charge. They reside in the nucleus alongside protons. Despite their lack of charge, neutrons play a crucial role in stabilizing the nucleus. The number of neutrons can vary within atoms of the same element, leading to different isotopes. For instance, carbon-12 and carbon-14 are both isotopes of carbon, with six protons each but differing in their number of neutrons.
Electrons: The Negative Charge
Electrons are negatively charged particles that orbit the nucleus in various energy levels or shells. Each electron carries a charge of -1. The arrangement of electrons around the nucleus follows specific rules known as quantum mechanics, determining the atom’s chemical properties and reactivity. Electrons are much lighter than protons and neutrons and occupy most of the atom’s volume, creating the electron cloud.

Forces Within the Atom
The stability of an atom is maintained by the balance of forces. The electromagnetic force causes the positively charged protons and negatively charged electrons to attract each other, holding the electrons in orbit around the nucleus. At the same time, the strong nuclear force binds protons and neutrons together in the nucleus, overcoming the repulsive electromagnetic force between the positively charged protons.
Quantum Mechanics: The Strange World of Subatomic Particles
At the subatomic level, the behavior of particles is governed by the principles of quantum mechanics. This branch of physics introduces concepts that can seem counterintuitive, such as wave-particle duality, which posits that particles like electrons exhibit both wave-like and particle-like properties. The Heisenberg Uncertainty Principle states that it’s impossible to simultaneously know both the exact position and momentum of a particle, highlighting the inherent probabilistic nature of quantum phenomena.
Why Understanding Atoms Matters
Understanding atoms and subatomic particles isn’t just an academic exercise; it has profound practical implications. Advances in atomic theory have led to the development of numerous technologies, from semiconductors in electronics to nuclear energy. Medicine benefits from atomic knowledge through techniques like MRI and radiation therapy. Moreover, the study of atoms continues to drive research in fields like materials science and quantum computing.
Conclusion
The exploration of atoms and subatomic particles reveals a world teeming with complexity and wonder. From the forces that bind the nucleus together to the probabilistic dance of electrons, the study of these tiny building blocks offers insights into the nature of reality itself. As our understanding of atoms deepens, so too does our ability to harness their potential, opening up new frontiers in science and technology.
Leave a Reply